[コンプリート!] difference between heterogeneous and homogeneous mixture class 9 284705-Difference between heterogeneous and homogeneous mixture class 9
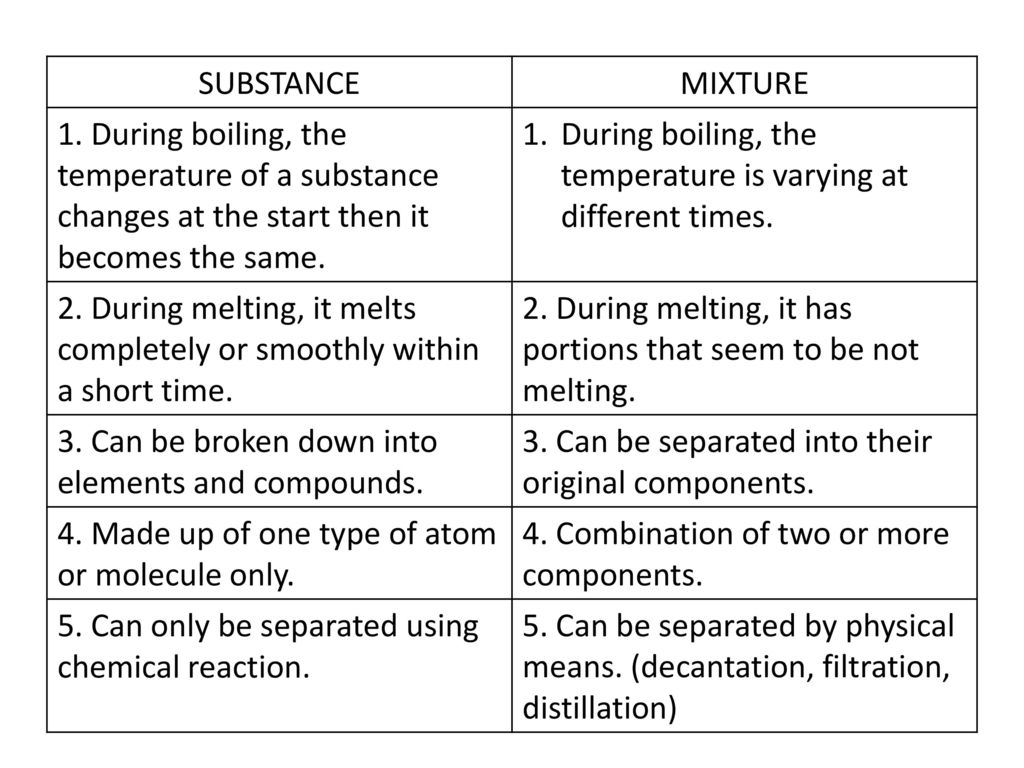
The Questions and Answers of What is the difference between heterogeneous mixtures and homogeneous mixtures ? Homogeneous Mixture Heterogeneous mixture They have uniform compositions They have nonuniform compositions The components of homogeneous mixtures are not physically distinct A heterogeneous mixture has physically distinct components They have no visible boundaries of separation between the constituents Differentiate between Homogeneous and Heterogeneous mixture with examples by Jaishree Gorane Leave a Comment List the points of difference between Homogeneous and Heterogeneous mixtures
Matter Substance Mixtures Elements Compounds Heterogeneous Homogeneous Ppt Download
Difference between heterogeneous and homogeneous mixture class 9
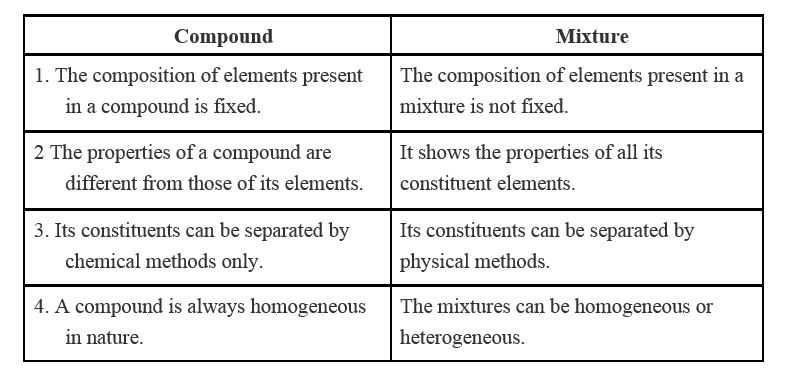
Difference between heterogeneous and homogeneous mixture class 9-(a) Sodium chloride from its solution in water (b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride (c) Small pieces of metal in the engine oil of a car (d) Different pigments from an extract of flower petals Difference between homogeneous and heterogeneous mixture 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a non uniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains physically distinct parts



1
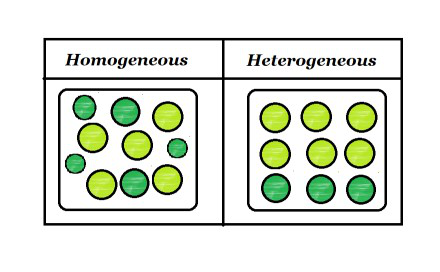
homogeneous means in which different constitute is mixedhetrogeneous means in which one constitute is mix This discussion on what is difference between homogeneous and heterogeneous mixture is done on EduRev Study Group by Class 9 Students The difference between heterogeneous and homogeneous mixtures is the degree at which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture where the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughoutMixtures are substances composed of two or more forms of matter You can separate them by physical methods Examples include a solution of salt and water, a
Homogeneous mixture Heterogeneous mixture 1 Uniform composition Nonuniform composition 2 The nondetectable boundary between solute and solventList the points of difference between homogeneous and heterogeneous mixture SOLUTION Q 3 Differentiate between homogeneous and heterogeneous mixtures with examples SOLUTION Refer Ans 2 above Q 4 How are sol, solution and suspension different from each other?Like us on Facebook https//wwwfacebookcom/k12mojoFollow us on twitter https//twittercom/K12MojoDownload our App https//playgooglecom/store/app
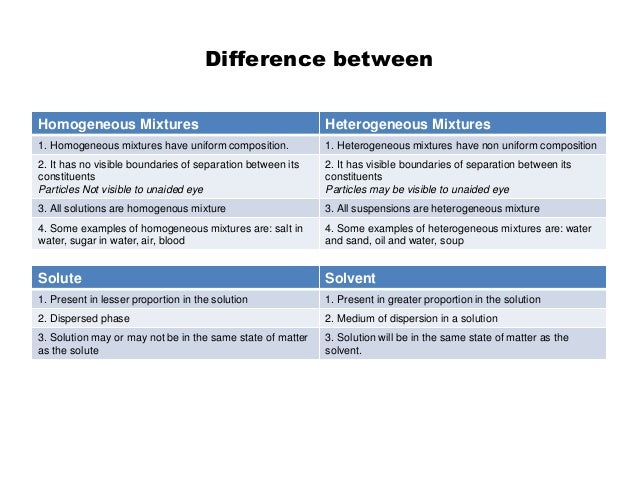
Homogeneous Mixture Heterogeneous Mixture; 1 Heterogeneous mixtures have non uniform composition 2 It has no visible boundaries of separation between its constituents 2 It has visible boundaries of separation between its constituents 3 Some examples of homogeneous mixtures are salt in water, sugar in water 3 Some examples of heterogeneous mixtures are water and sand, oil andA homogeneous mixture is one in which the composition of the components is the same throughout the body of the mixture But , in a heterogeneous mixture the composition of the components is not same throughout
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures



Is Matter Around Us Pure Practically Study Material
As a homogeneous mixture has two or more distinct phasesMixtures which do not have uniform composition throughout are called Heterogeneous Mixture For example – mixture of soil and sand, mixture of sulphur and iron fillings, mixture of oil and water etc The boundaries of constituent particles of a homogeneous mixture can be identified easily;#9thchemistry #subscriberHi, Iam Muhammed Yaseen TKToday we can look difference between homogeneous mixture and heterogeneous mixture ഒമ്പതാം ക്ലാസിലെ ക




Matter Substance Mixtures Elements Compounds Heterogeneous Homogeneous Ppt Download




Chemistry For Kids Chemical Mixtures
Is done on EduRev Study Group by Class 9 Students The Questions and Answers of What is difference between homogeneous and heterogeneous mixture ?Explain the difference between a homogeneous mixture and a heterogeneous mixture One useful way of organizing our understanding of matter is to think of a hierarchy that extends down from the most general and complex to the simplest and most fundamental (Figure \(\PageIndex{1}\)) Mixture of Oil and Water is a heterogeneous Mixture We are able to see oil and water clearly separately in the mixture Example 2 Mixture of Salt (Sodium Chloride) and Iron filings is a heterogeneous mixture The particles of salt and Iron filings can be seen and distinguished easily Difference between Homogeneous and Heterogeneous mixtures



1




Homogenous Vs Homogeneous What S The Difference Writing Explained
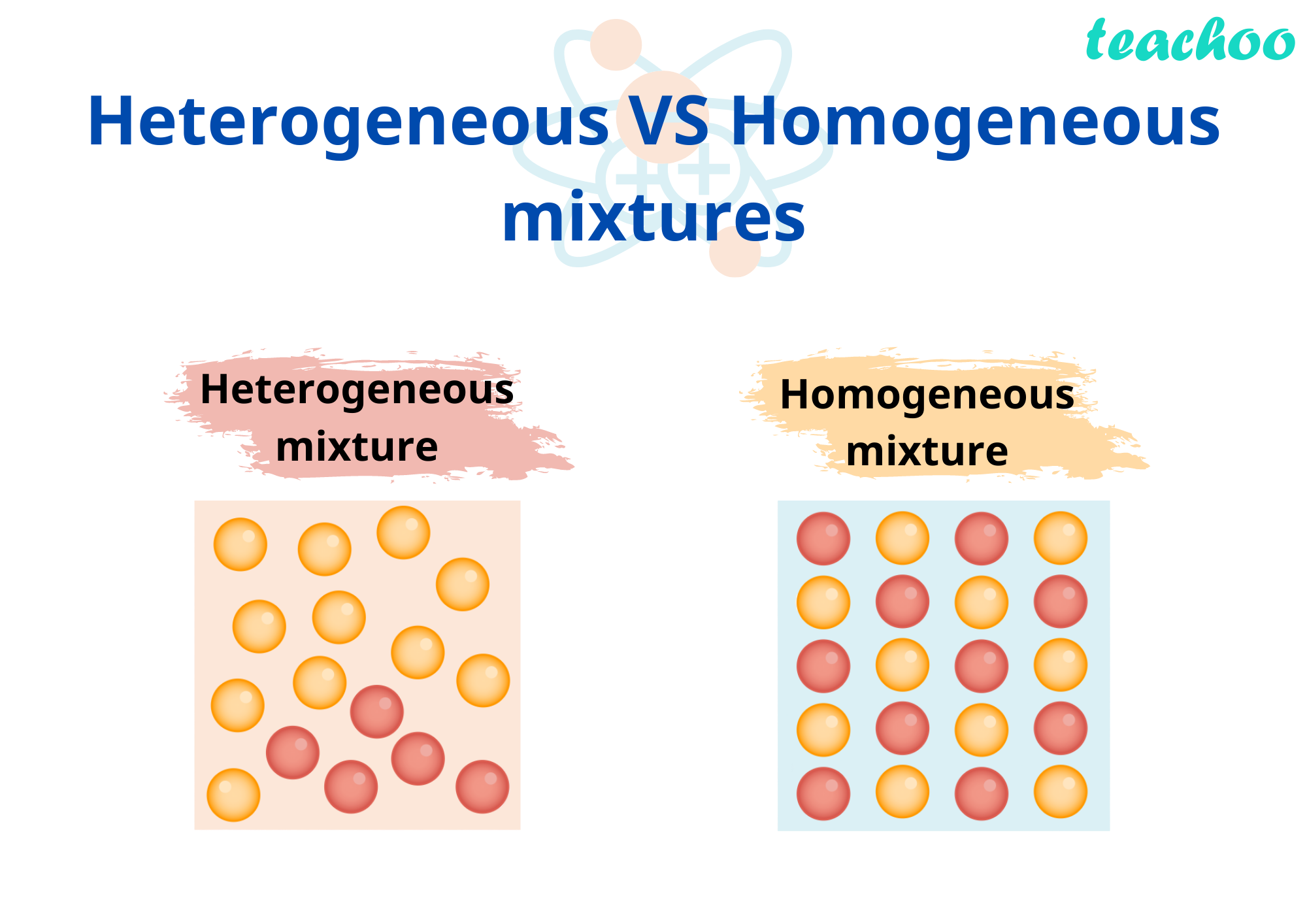
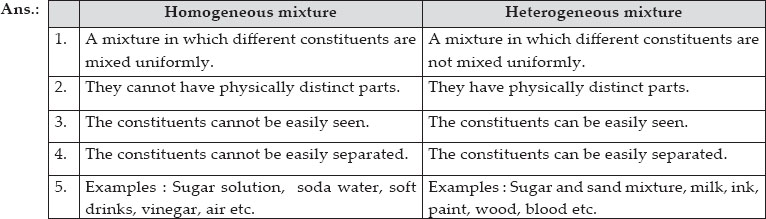
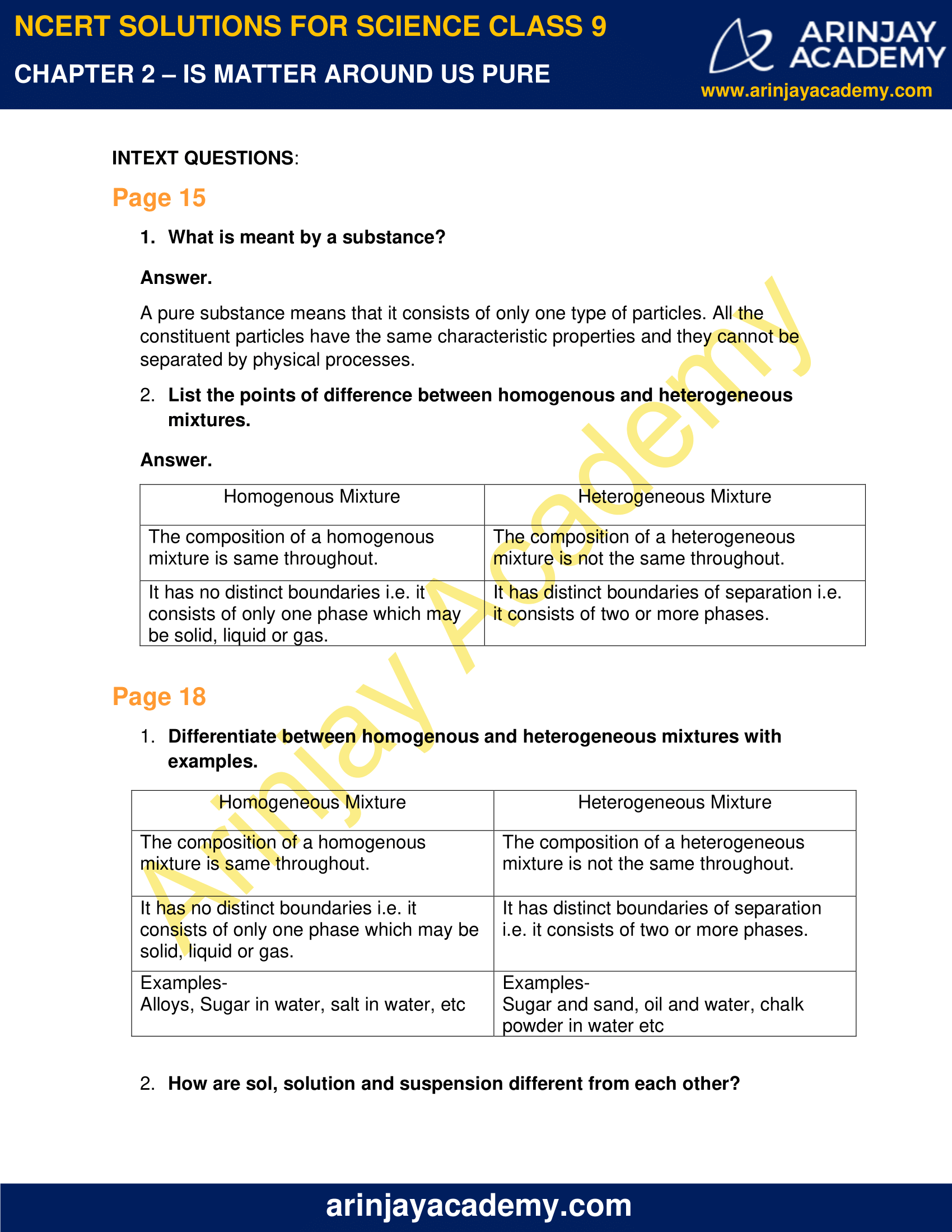
A homogeneous mixture has the same uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquid, and solid Homogeneous mixture Heterogeneous mixture Its constituents are uniformly distributed all over the mixture It's constituents are not distributed uniformly There are no visible boundaries of separation There are distinct and visible boundaries of separation in most of the cases Components are not visible to the naked eye Components can be seen easily Question 1 Page 18 Chapter 2 Class 9 Is Matter Around Us Pure (Term 1) Last updated at by Teachoo Differentiate between homogeneous and heterogeneous mixtures with examples




What Is Difference Between Heterogeneous And Homogeneous Brainly In




The Sea Water Can Be Classified As A Homogeneous As Well As Heterogeneous Mixture Comment
Answer Difference between homogeneous and heterogeneous mixture are 1) A homogeneous mixture has a uniform composition throughout its mass whereas a heterogeneous mixture has a non uniform composition 2) A homogeneous mixture does not contain physically distinct parts whereas a heterogeneous mixture contains physically distinctAlso Read Difference Between Compound And Mixture Difference Between Homogenous And Heterogeneous Mixture In Tabular Form BASIS OF COMPARISON HOMOGENOUS MIXTURE HETEROGENEOUS MIXTURE Nature In this type of mixtures, the components are uniformly distributed throughout the mixture HOMOGENEOUS MIXTURE HETEROGENEOUS MIXTURE 1) Those mixtures in which the substances are completely mixed together and are indistinguishable from one another Those mixtures in which the substances remain separate and one substance is spread throughout the other 2) They have uniform composition throughout its mass




A State The Main Points Of Difference Between Homogeneous And Heterogeus And Heterogeneous Brainly In




Solved What Is The Difference Between Homogeneous And Heterogeneous Matter Classify Each Of The Following As Homogeneo
Difference between Homogeneous and Heterogeneous Mixture Homogeneous mixture Heterogeneous mixture It has a uniform composition It has a nonuniform composition It has only one phase There are two or more phases It can't be separated out physically It can be separated out physicallyThe difference between heterogeneous and homogeneous mixture is the degree to which the materials are mixed together and the uniformity of their composition example of homogeneous is blood plasma heterogeneous is sand in water HOMOGENEOUS MIXTURE A homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample HETEROGENOUS MIXTURE A heterogeneous mixture is simply any mixture that is not uniform in composition – it's a nonuniform mixture of smaller constituent parts




Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples Brainly In



Examples Of Solution Mixtures
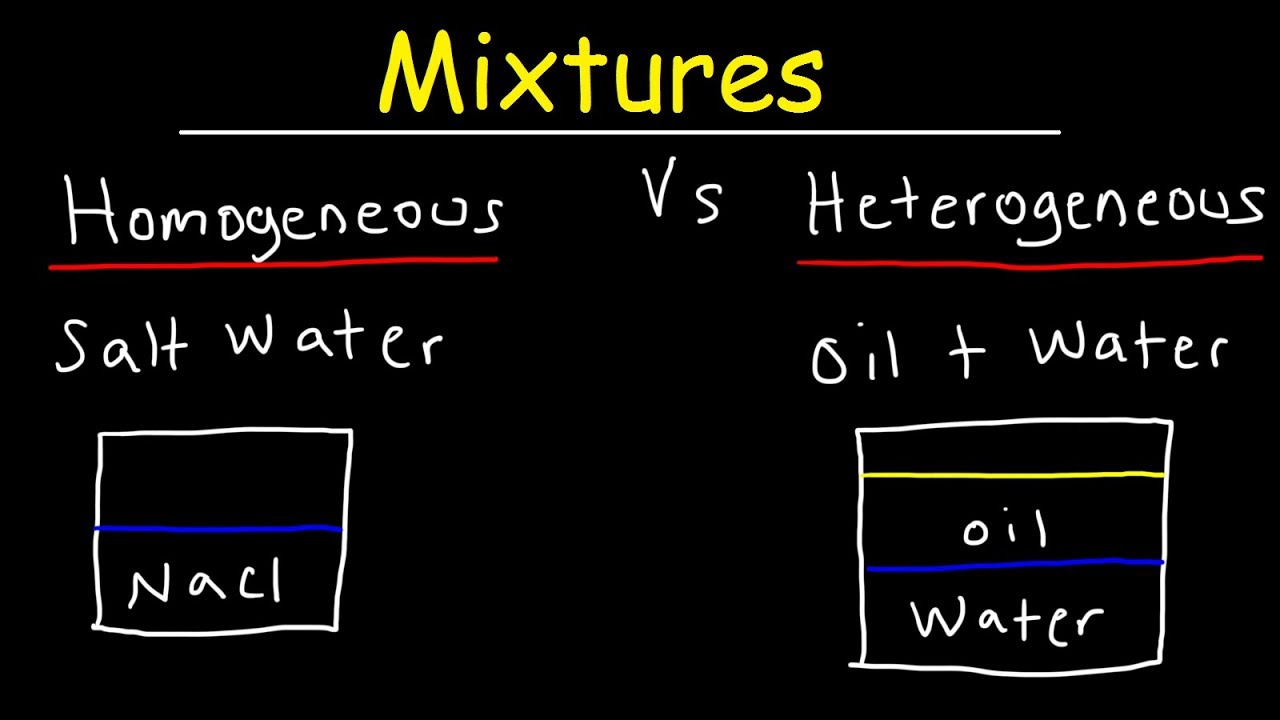
A homogeneous mixture is a mixture having a uniform composition throughout the mixture For example salt in water, sugar in water, copper sulphate in water A heterogeneous mixture is a mixture having a nonuniform composition throughout the mixture For example sodium chloride and iron fillings, salt and sulphur, oil and waterThis video is in simple language about Difference between homogenous and heterogeneous mixturesClass 9Chapter 2Is Matter Around Us Pure Differentiate between Homogeneous and Heterogeneous mixture with examples Difference Between Plant Cell and Animal Cell for Class 9




Selina Icse Solutions For Class 9 Chemistry Elements Compounds And Mixtures A Plus Topper
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures
What is difference between homogeneous mixture and heterogeneous mixture Class 9?Homogenous mixtures generally have a uniform composition throughout the mixture whereas Heterogeneous mixtures have composition which may vary from point to pointQ List the points of difference between homogeneous and heterogeneous mixture Answer Homogeneous mixture Heterogeneous mixture 1 A mixture




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture
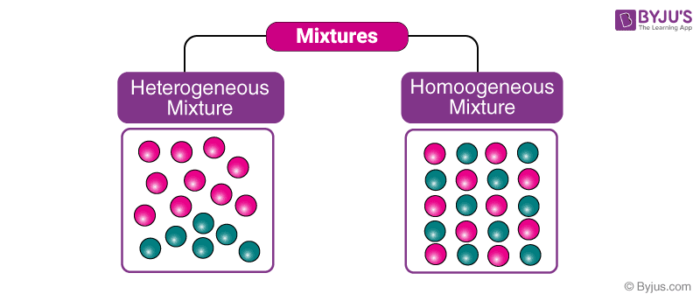
Are solved by group of students and teacher of Class 9, which is also the largest student community of Class 9 Homogeneous mixtures are composed entirely of one phase (for instance, all liquid or all solid) A heterogeneous mixture is composed to mixed phases (such as a solid in a liquid, or a liquid and gas) Homogeneous Mixtures salty water (where the salt is completely dissolved) brewed tea or coffee soapy water Heterogeneous MixturesCheerios Trail mix Trail mix is HETEROGENEOUS Homogeneous Mixtures Heterogeneous Mixtures centrifugation coagulation distillation evaporation filtration hand picking magnetic separation sieving winnowing sedimentation



Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




Heterogeneous And Homogeneous Mixture Differences Videos Examples
46 cm 3 of methyl alcohol is dissolved in 252 g of water Calculate (i) % by mass of methl alcohol (ii) mole fraction of methyl alcohol and water (Given density of methyl alcohol = gcm − 3 and C = 12, H = 1, O = 16) i) 1268 ii) ,Background Music Credit Alan Walker Fade NCS Release Video link https//youtube/bM7SZ5SBzyY1 Mixtures that have uniform composition Mixtures that do not have uniform composition throughout 2 Boundary of separation could not be seen Boundary of separation of constituent particles is clearly visible 3 Particles are not indistinguishable Particles can be physically distinguishable 4



1



Homogeneous Mixture And Heterogeneous Mixture Ncert Books
Are solved by group of students and teacher of Class 9, which is also the largest student community of Class 9After highlighting the differences between mixtures and compounds, we will be giving a brief description of mixtures and compounds Mixture When we mix two or more substances together, whose ratio is not fixed such that no chemical reaction takes place, the substance formed is a mixtureSoda water, lemonade, a mixture of salt in water are a few examples of homogeneous mixtures Heterogeneous Mixtures – Those mixtures which do not have a uniform composition entirely are heterogeneous mixtures A mixture of oil and water, soil and sand are heterogeneous mixtures as they don't have a uniform composition Difference Between




Classify Mixtures As Homogeneous Or Heterogeneous Worksheet



Differentiate Between Mixtures And Compound Chemistry Topperlearning Com Wzmi1566
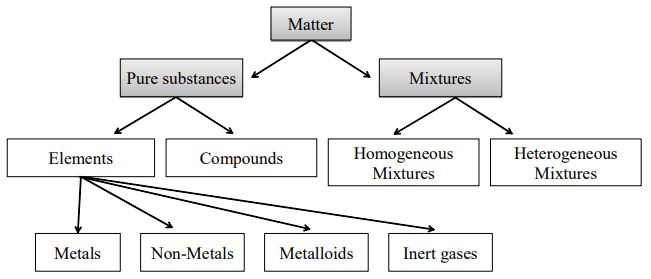
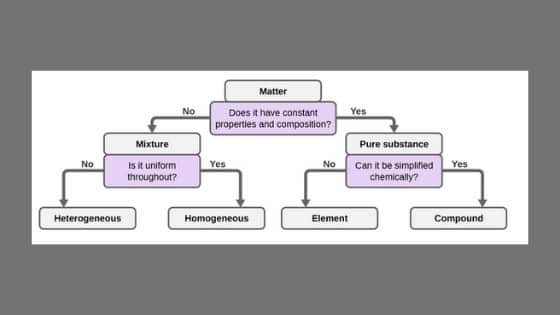
11 Which separation techniques will you apply for the separation of the following? Substances which have a specific composition and cannot be separated into any constituents are called pure substances Pure substances are further divided into elements and compounds The combination of two or more pure substances is called a mixture Mixtures can be classified into two types viz heterogeneous and homogeneous mixturesHeterogeneous mixture (I) Homogeneous mixtures have uniform composition throughout the mixture (II) The whole mixture is in same phase (III) Components are not visible to the naked eye (IV) Components cannot be separated easily Eg Sugar Water → Sugar solution (I) Heterogeneous mixture have composition which may vary from point to point




Effective And Creative Lesson Plans For Teachers By Teacher Lesson Plan Of Difference Between Solutions And Suspensions General Science Grade Vi




Difference Between Homogenous And Heterogenous Mixture
Heterogeneous mixtures have nonuniform composition What type of mixtures are these?Solution Homogeneous Mixtures Heterogeneous mixtures The elements and compounds are uniformly mixed The elements and compounds are not uniformly mixed The properties of the mixture are the same in all compositions The properties differ at different compositions Copper and Zinc are two solids which make brass, which is solid The difference between compounds and mixtures can be seen from the fact that compounds are generally homogeneous When two or more atoms of different elements join together, the resulting bond is a compound



Difference Between Compound And Mixture Definition Characteristics Types Of Bonding




10 Examples Of Mixtures
This discussion on What is difference between homogeneous and heterogeneous mixture ? A mixture of alcohol and water is homogeneous while that of oil and water is heterogeneous Explain asked in Class IX Science by ashu Premium ( 930 points) The homogeneous mixture is the combination of two or more pure substance in such a uniform manner that each of the substance is indistinguishable from the other substance, whereas the pure substances in the heterogeneous mixture are not uniformly distributed, and it results in the formation of nonuniform composition




What Is The Difference Between Heterogeneous Mixture Vs Homogenous Mixture Brainly Com




Homogeneous Mixture Examples Found At Home




Examples Of Heterogeneous Mixtures Types Made Simple




Difference Between Homogeneous Mixture And Heterogeneous Mixture



Is Matter Around Us Pure




Solutions Unit 3 Solution It Is A Homogeneous Mixture That Is Formed When A Substance Is Dissolved In Another Substance Ppt Download




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube



Separating Mixtures Lesson Teachengineering



Homogeneous Vs Heterogeneous Matter Worksheet Worksheet List




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




Food Chemistry Distinguish Between Pure Substances And Mixtures Compare Homogeneous And Heterogeneous Mixtures Define Solutions Distinguish Ppt Download




What Is A Heterogeneous Mixture Definition And Examples




Identify The Following As Homogeneous And Heterogeneous Mixtures I Sugar Dissolved In Water Ii Oil And Water




Unit Matter Objectives Lesson 3 Of 4 You Will Understand The Difference Between An Element And A Compound You Will Learn How Bonds Form Between Atoms Ppt Download



Is Sugar A Homogeneous Or Heterogeneous Mixture Chemistry Point



2




Homogeneous And Heterogeneous Mixtures Card Sorting Activity By Elly Thorsen




List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures




1 Differentiate Between Homogon Eous Aid Heterogeneous Mixt Scholr




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




List 5 Difference Between Homogenous Anf Heterogenous Mixture Brainly In




Difference Between Homogeneous And Heterogeneous Material Youtube




Chapter 1 Measurements In Chemistry Chemistry




Classify Each Of The Following As A Homogeneous Or Heterogeneous Mixture Soda Water




List The Point Of Difference Between Homogeneous And Heterogeneous Mixture Science Is Matter Around Us Pure Meritnation Com



What Does It Mean When A Mixture Is Heterogeneous




Solved What Is The Difference Between Homogeneous And Heterogeneous Matter Classify Each Of The Following As Homogeneo




Homogeneous Or Heterogeneous Mixtures Practice Worksheet




Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples Youtube




Homogeneous Mixture Examples Chemistry




Write The Difference Between Homogeneous And Heterogeneous Solution With An Example Brainly In




Mixtures Worksheet




What Is A Homogeneous Mixture Definition And Examples



1



Mixture
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Homogeneous Mixture Examples In Daily Life



Homogeneous And Heterogeneous Mixtures Geeksforgeeks



Ncert Solutions For Class 9 Science Chapter 2 Arinjay Academy




Q2 Differentiate Between Homog Lido




What Is Suspension In Science Definition Types Examples Video Lesson Transcript Study Com



Betrained In Evergreen 9 Science Is Matter Around Us Pure Solution




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




Difference Between Homogenous And Heterogenous Mixture




Types Of Mixtures Video Khan Academy



Ncert Solutions For Class 9 Science Chapter 2 Is Matter Around Us Pure



Heterogeneous And Homogeneous Mixture Differences Videos Examples
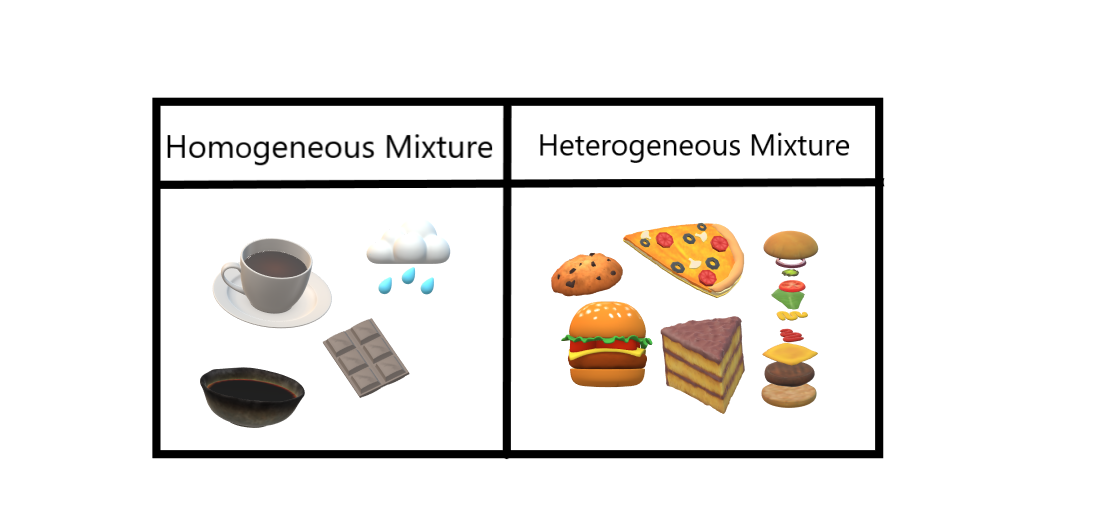



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Mixtures Online Worksheet




Heterogeneous And Homogeneous Mixture Differences Videos Examples




Is Matter Around Us Pure Ncert Solutions For Class 9th Science Chapter 2 Imperial Study




Heterogeneous And Homogeneous Mixtures Worksheet Elementary Science Experiments Middle School Science Experiments Science Experiments



Ch 3




Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Exam Heterogeneous Mixture Chemistry Basics Biology Facts



Teachbcdb tf Ca Download 1154 Filename Sd71webchemistryg6 Pdf



Http Sites Isdschools Org Grade6 Remote Learning Resources Useruploads 05 11 Science6 Schimmelsmartwynn May13 Pdf




List The Point Of Differences Between Homogeneous And Heterogeneous Mixture Brainly In




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube




Month April Padhai Guru




Difference Between Sol Solution And Suspension Compare The Difference Between Similar Terms




Homogeneous And Heterogeneous Mixtures Editable Venn Diagram Template On Creately



Q Tbn And9gcss8oj4wp26w4gdoy1uuuzqiv502ma6xsk3cgpr6wqtj53hz7nw Usqp Cau




Air Is Homogeneous Mixture Or Heterogeneous Mixture Edurev Class 9 Question




Give Three Differences Between Homogeneous Mixture And Heterogeneous Mixture Brainly In




Difference Between Homogeneous And Heterogeneous Mixture Brainly In




Cbse Class 9 Science Practical Skills Mixture And Compound Cbse Sample Papers




Mixture Homogeneous And Heterogeneous Mixtures Ck 12 Foundation




Ncert Solutions For Class 9 Science Chapter 2 Is Matter Around Us Pure



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora




What Is Homogeneous Mixture In Hindi




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards




Mixtures Homogeneous And Heterogeneous Mixtures Ppt Video Online Download



Homogeneous And Heterogeneous Mixture Nine Science



Http Grade7byng Pbworks Com W File Fetch S7 ptm Mixtures disted lessons 1 3 Pdf




Homogeneous Mixture Examples In Kitchen
コメント
コメントを投稿